I. Introduction
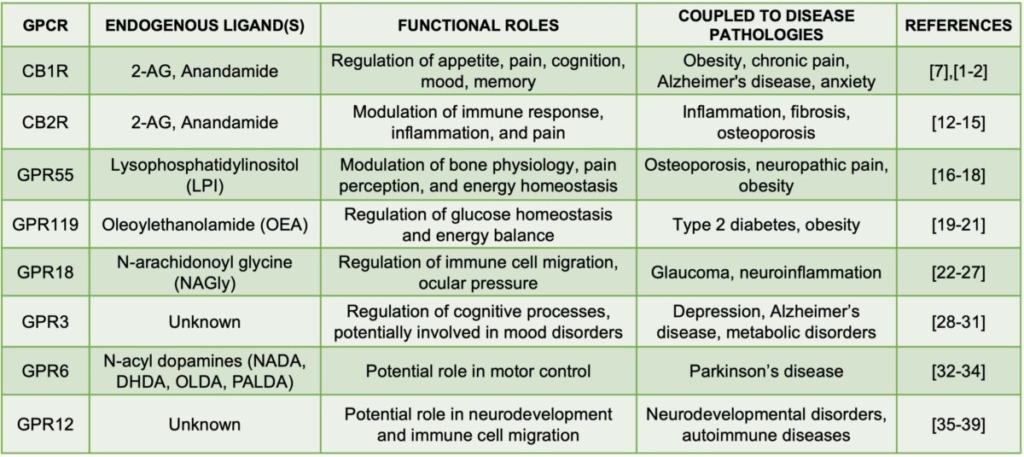
The endocannabinoid system (eCBome) is a vital biological entity that governs a plethora of physiological functions, from mood regulation to immune response [1-2]. Built on a foundation of cannabinoid receptors, endogenous ligands, and the enzymes responsible for their synthesis and degradation, the eCBome functions through extensive interactions with G-protein Coupled Receptors (GPCRs) [2]. As the most abundant membrane proteins in the human genome, GPCRs enable the transmission of extracellular signals into the cell, thereby regulating a multitude of cellular functions [3]. Key GPCRs within the eCBome include CB1R, CB2R, GPR55 and several more[4]. Their interactions with endogenous cannabinoids and related compounds is of profound physiological importance, and holds immense therapeutic potential. Aberrations in these interactions are linked to numerous pathologies, such as obesity, type 2 diabetes, neurological disorders, and inflammatory diseases. [5][6]. Hence, comprehensive knowledge of eCBome GPCR pharmacology is crucial for devising impactful therapeutic strategies to tackle a wide range of diseases. In this review, we delve into the fascinating roles of various eCBome GPCRs and their implications in human pathologies.
II. eCBome and Cannabinoid Receptors: CB1R and CB2R
The eCBome hinges on two key cannabinoid receptors: CB1R and CB2R. These receptors, which are part of the GPCR family, respond to endogenous ligands such as 2-arachidonoylglycerol (2-AG) and anandamide, but each plays unique roles in human physiology and pathology. [7].
CB1R, primarily found in the brain, regulates appetite, cognition, mood, sleep, and pain [1][2]. Disruption of CB1R signaling can lead to various pathologies, including obesity, chronic pain, Alzheimer’s disease, and anxiety, underscoring the therapeutic potential of modulating CB1R signaling. [8][9][10][11].
Conversely, CB2R, largely expressed in the immune system, influences immune response and inflammation [12]. By modulating CB2R signaling, we can potentially control inflammation, fibrosis, and conditions such as osteoporosis. [13][14][15]. Given the role inflammation plays in many diseases, CB2R emerges as a promising target for therapeutic intervention.
Recognizing the integral roles of CB1R and CB2R in the eCBome, and the range of diseases influenced by their dysregulation, it’s clear that continued research in this area is crucial. A deeper understanding of these receptors could open up new avenues for developing novel therapeutic strategies.
III. eCBome GPCRs Beyond CB Receptors: GPR55 and GPR119
The spectrum of eCBome GPCRs extends beyond CB receptors, incorporating ‘non-classical’ cannabinoid receptors such as GPR55 and GPR119.
GPR55, while not traditionally recognized as a cannabinoid receptor, has attracted substantial interest due to its interaction with several cannabinoids. Its activation by the endogenous ligand Lysophosphatidylinositol (LPI) impacts bone physiology, pain perception, and energy homeostasis [16][17][18]. Furthermore, GPR55’s role in diseases like osteoporosis, neuropathic pain, and obesity suggests promising new therapeutic strategies [16][17][18].
Similarly, GPR119, primarily located in the gastrointestinal tract and pancreatic β-cells, contributes to the eCBome’s functions [19][20][21]. Activation of GPR119 by the ligand Oleoylethanolamide (OEA) regulates glucose homeostasis and energy balance, which are critical for metabolic health. Disorders in GPR119 signaling are linked with Type 2 diabetes and obesity, thereby showcasing its potential as a therapeutic target [19][20][21].
The exploration of GPR55 and GPR119 highlights the multifaceted nature of the eCBome, broadening our understanding beyond traditional cannabinoid receptors.
IV. eCBome GPCR: GPR18 and Its Roles in Immune Regulation
The complexity of the eCBome increases with the involvement of GPR18, a GPCR with a multifaceted role that has only recently been recognized.
GPR18 is present in various tissues, from the central nervous system to immune cells and peripheral tissues, indicating its extensive physiological reach [22][23]. The endocannabinoid-like compound N-arachidonoyl glycine (NAGly) is primarily identified as GPR18’s endogenous ligand, though other cannabinoids have been shown to activate it, cementing its role in the eCBome [24].
GPR18 plays diverse and significant roles, particularly in immune regulation. Its activation influences the migration of immune cells, potentially affecting inflammation and wound healing [25]. Moreover, it seems to modulate pain perception, adding another dimension to the eCBome’s role in nociception [22].
Dysregulation of GPR18 has been associated with several pathologies, including glaucoma, hypertension, and neuroinflammatory diseases, highlighting its crucial role in maintaining physiological homeostasis [26][27]. Our expanding understanding of GPR18 further deepens the complexity of the eCBome, indicating future research directions in the pharmacology of GPCR-targeted therapies
V. eCBome GPCRs: GPR3, GPR6, and GPR12 in Neurological Functions
The eCBome’s influence extends into neurological functions through GPCRs like GPR3, GPR6, and GPR12, each playing unique yet critical roles in this complex system. Phylogenetic analyses have shown that these receptors are the three most closely related GPCRs to CB1 and CB2 in the human genome, suggesting that they might interact with fairly similar ligands [28]. In 2019, all three receptors were discovered to be affected by Cannabidiol (CBD) as an inverse agonist. The first endogenous ligands for GPR6 were described only a few years ago in 2020 and were discovered to be N-acyl dopamines, functioning as endogenous inverse agonists, attenuating the high constitutive activity of GPR6 [34].
GPR3, highly expressed in the brain, influences cognitive processes and mood regulation within the eCBome [29]. Emerging research suggests that high intrinsic constitutive activation of GPR3 might be linked to increased amyloid-beta production, implying a potential role in Alzheimer’s disease [30]. GPR3 signaling is also implicated in mood disorder regulation, adding to the complexity of the eCBome [31].
GPR6, though relatively understudied, shows potential involvement in motor control due to its abundant expression in the striatum, a brain region pivotal for voluntary movement, reward pathways, learning, and memory [32,33]. Striatal structures play a key role in many neurodegenerative and neuropsychiatric disorders, including Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, schizophrenia, autism, and drug addiction. Recent discoveries suggest that dysregulation of GPR6 is linked to Parkinson’s disease, making it an exciting focus for future research [35].
GPR12, another GPCR, has compelling implications for neurodevelopment and immune functions [36]. It is predominantly expressed in brain regions associated with learning and memory [37]. Dysregulation of GPR12 has been linked to disorders like autism and schizophrenia, underscoring its importance in neurological health [38,39].
Together, the exploration of these GPCRs sheds light on the eCBome’s extensive role in neurological functions, presenting new research pathways and therapeutic potential.
VI. Conclusion
The endocannabinoid system (eCBome) has positioned itself as a key regulator of numerous physiological processes via its array of G-protein-coupled receptors (GPCRs). Central to this system are the cannabinoid receptors CB1R and CB2R, whose roles in neuropsychiatric and immune-related conditions are well-established [4,8]. However, the discovery of other eCBome GPCRs, such as GPR55, GPR119, GPR18, GPR3, GPR6, and GPR12, has significantly broadened our understanding of the potential influence of the eCBome on human health [2,5,22,29,34,36].
Each of these GPCRs exhibits unique characteristics in terms of ligand specificity, distribution, and functional roles, underscoring the eCBome’s diverse roles in health and disease. For instance, GPR55’s involvement in energy homeostasis and inflammatory responses, GPR119’s role in metabolic disorders, and GPR18’s influence on immune regulation, have provided fresh perspectives on their respective physiological and pathological realms [2,5,22]. Similarly, GPR3, GPR6, and GPR12, primarily found in the brain, have pointed to potential impacts on cognitive processes, mood, motor control, and neurodevelopment [29,34,36].
Uncovering these receptors’ functions highlights the promising potential of eCBome GPCRs as therapeutic targets for a range of conditions, from metabolic disorders to neurodegenerative diseases [1,2]. Future research should aim to fully elucidate the signaling pathways and range of ligands for these GPCRs, which could guide the development of more selective and effective therapeutic strategies. Yet, it is crucial to emphasize that a comprehensive understanding of eCBome GPCR pharmacology is indispensable for capitalizing on their therapeutic potential, reaffirming the need for continued exploration in this field.
Stefan Broselid, Ph.D.
Editor-In-Chief, Aurea Care Medical Science Journal
VII. References
- Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16(1):9-29. doi:10.1038/s41582-019-0284-z
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475-490. doi:10.1016/j.cmet.2013.03.001
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(12):829-842. doi:10.1038/nrd.2017.178
- Pertwee RG. Endocannabinoids and Their Pharmacological Actions. Handb Exp Pharmacol. 2015;231:1-37. doi:10.1007/978-3-319-20825-1_1
- Overton HA, Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167-175. doi:10.1016/j.cmet.2006.02.004
- Idris AI, Ralston SH. Role of cannabinoids in the regulation of bone remodeling. Front Endocrinol (Lausanne). 2012;3:136. doi:10.3389/fendo.2012.00136
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199-215. doi:10.1038/sj.bjp.0707442
- Di Marzo, V. (2008). Targeting the endocannabinoid system: to enhance or reduce? Nature Reviews Drug Discovery, 7(5), 438-455. doi:10.1038/nrd2553.
- Cota D, Marsicano G, Tschöp M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423-431. doi:10.1172/JCI17725
- Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol. 2007;5(2):73-80. doi:10.2174/157015907780866884
- Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3353-3363. doi:10.1098/rstb.2011.0381
- Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:e3. Published 2009 Jan 20. doi:10.1017/S1462399409000957
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156(3):397-411. doi:10.1111/j.1476-5381.2008.00048.x
- Turcotte C, Blanchet MR, Laviolette M, Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci. 2016;73(23):4449-4470. doi:10.1007/s00018-016-2300-4
- Bab I, Zimmer A, Melamed E. Cannabinoids and the skeleton: from marijuana to reversal of bone loss. Ann Med. 2009;41(8):560-567. doi:10.1080/07853890903121025
- Henstridge CM, Balenga NA, Ford LA, et al. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23(1):183-193. doi:10.1096/fj.08-108670
- Whyte LS, Ryberg E, Sims NA, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009;106(38):16511-16516. doi:10.1073/pnas.0902743106
- Hryhorowicz S, Kaczmarek-Ryś M, Zielińska A, Scott RJ, Słomski R, Pławski A. Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease – A Systematic Review. Front Immunol. 2021;12:790803. Published 2021 Dec 22. doi:10.3389/fimmu.2021.790803
- Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58(5):1058-1066. doi:10.2337/db08-1237
- Overton HA, Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167-175. doi:10.1016/j.cmet.2006.02.004
- Li H, Fang Y, Guo S, Yang Z. GPR119 agonists for the treatment of type 2 diabetes: an updated patent review (2014-present). Expert Opin Ther Pat. 2021;31(9):795-808. doi:10.1080/13543776.2021.1921152
- Kohno M, Hasegawa H, Inoue A, et al. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347(3):827-832. doi:10.1016/j.bbrc.2006.06.175
- McHugh D, Hu SS, Rimmerman N, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. Published 2010 Apr 2. doi:10.1186/1471-2202-11-44
- Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(1):4-15. doi:10.1016/j.pnpbp.2012.01.001
- Takenouchi R, Inoue K, Kambe Y, Miyata A, Kitajima S, Watanabe Y. N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem Biophys Res Commun. 2012;418(2):366-371. doi:10.1016/j.bbrc.2012.01.017
- Caldwell MD, Hu SSJ, Viswanathan S, Bradshaw H, Kelly MEM, Straiker A. A GPR18-based signalling system regulates IOP in murine eye. Br J Pharmacol. 2013;169(4):834-847. doi:10.1111/bph.12136
- Ho WS, Kelly ME. Cannabinoids in the Cardiovascular System. Adv Pharmacol. 2017;80:329-366. doi:10.1016/bs.apha.2017.05.004
- Laun AS, Shrader SH, Brown KJ, Song ZH. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2019;40(3):300-308. doi:10.1038/s41401-018-0031-9
- Valverde O, Célérier E, Baranyi M, et al. GPR3 receptor, a novel actor in the emotional-like responses. PLoS One. 2009;4(3):e4704. doi:10.1371/journal.pone.0004704
- Thathiah A, Horré K, Snellinx A, et al. β-arrestin 2 regulates Aβ generation and γ-secretase activity in Alzheimer’s disease. Nat Med. 2013;19(1):43-49. doi:10.1038/nm.3023
- Watkins LR, Orlandi C. Orphan G Protein Coupled Receptors in Affective Disorders. Genes (Basel). 2020;11(6):694. Published 2020 Jun 24. doi:10.3390/genes11060694
- Laun AS, Song ZH. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun. 2017;490(1):17-21. doi:10.1016/j.bbrc.2017.05.165
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543-554. doi:10.1016/j.neuron.2008.11.005
- Shrader SH, Song ZH. Discovery of endogenous inverse agonists for G protein-coupled receptor 6. Biochem Biophys Res Commun. 2020;522(4):1041-1045. doi:10.1016/j.bbrc.2019.12.004
- Oeckl P, Hengerer B, Ferger B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson’s disease. Exp Neurol. 2014;257:1-9. doi:10.1016/j.expneurol.2014.04.010
- Allende G, Chávez-Reyes J, Guerrero-Alba R, Vázquez-León P, Marichal-Cancino BA. Advances in Neurobiology and Pharmacology of GPR12. Front Pharmacol. 2020;11:628. Published 2020 May 8. doi:10.3389/fphar.2020.00628
- Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth
- Zhao M, Ma J, Li M, et al. Different responses to risperidone treatment in Schizophrenia: a multicenter genome-wide association and whole exome sequencing joint study. Transl Psychiatry. 2022;12(1):173. Published 2022 Apr 28. doi:10.1038/s41398-022-01942-w
- Frank E, Wu Y, Piyaratna N, et al. Metabolic parameters and emotionality are little affected in G-protein coupled receptor 12 (Gpr12) mutant mice. PLoS One. 2012;7(8):e42395. doi:10.1371/journal.pone.0042395

